

Clausius showed that for every thermodynamic system there exists a function of the state of the system, its entropy denoted by S. The modern statement of this principle of dissipation is based upon the notion of entropy, introduced by Clausius in 1865. Essentially, the second law states that heat can never pass from a colder to a warmer body without some other related change occurring at the same time. At least two heat sources at different temperatures are needed as shown by Carnot's argument (sometimes one of the sources may be naturally supplied by the environment).
Whereas one can easily perform work to heat up the system, it is not always enough to supply heat to increase the mechanical energy. The second law of thermodynamics indicates that not all the processes compatible with the first law can actually occur. It follows from this that a body has a temperature distribution which is a function of space and time, T = T( x, y, z, t), and that the thermal field within the solid is constructed by the superposition of an infinite number of isothermal surfaces which never intersect, lest some point of intersection in space be simultaneously at two or more temperatures which is impossible. In addition, the first law of thermodynamics requires that the thermal energy is conserved in the absence of heat sources or sinks in the body. In essence, this is a statement that a thermal gradient must exist in the solid and that heat flows down the thermal gradient. The second law of thermodynamics requires that heat is transferred from one body to another body only if the two bodies are at different temperatures, and that the heat flows from the body at the highest temperature to the body at the lowest temperature. George Alanson Greene, in Encyclopedia of Physical Science and Technology (Third Edition), 2003 I.B Fundamental Law of Heat Conduction It is postulated that this function cannot decrease with time for a closed system. The feature of this function is related to the spectrum of microstates. It is the second law of thermodynamics that from a formal point of view allows us to introduce a macroscopic function: entropy S. In terms of the relationships between the microscopic and macroscopic states of the system, the second law, to some extent, subordinates the status of macrostates to only a certain set of microstates. The second law underscores the crucial irreversibility of the thermodynamics of all processes of energy conversion and directs this irreversibility to the thermal degree of freedom as the most sustainable energy form.
It reveals the reaction of the system, describes a macroscopic interaction as a way to change the nonequilibrity, and highlights the special status of the thermal degree of freedom as the most equilibrated (stable one), thereby selecting the thermal energy, both qualitatively and quantitatively.

The second law of thermodynamics reveals the properties of reversibility/stability or irreversibility/instability of a process of interaction of one or another degree of freedom or that of another way of energy exchange. Entropy is related to quantum microstates of a system via probability of a macrostate.Īdam Moroz, in The Common Extremalities in Biology and Physics (Second Edition), 2012 1.1.8 Macroparameters: Universal Fatality of the Processes-The Second Law of Thermodynamics and the Hierarchy of Energy Enthalpy and entropy changes are calculated for an isobaric melting of ice. Reversible and irreversible expansion of an ideal gas are illustrated.
Second law of thermodynamics equation plus#
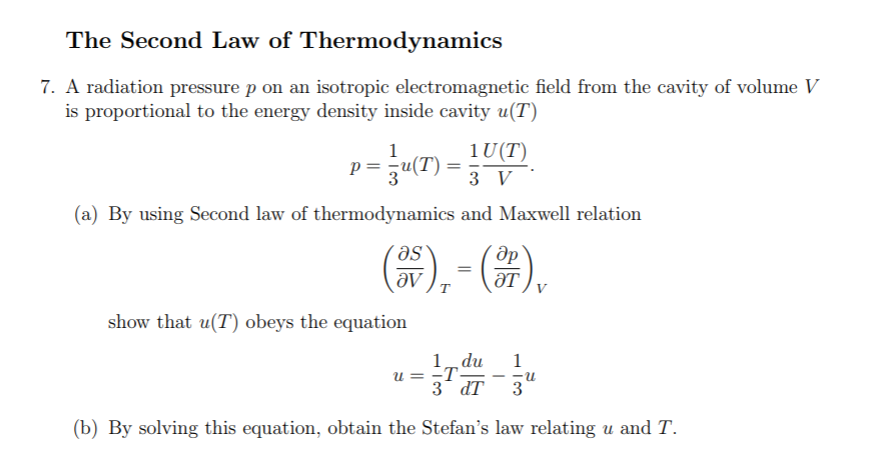
The second law plus the first law establish a fundamental equation to calculate entropy changes as a function of state. We relate entropy to its historical roots including other postulates and the Carnot cycle for an ideal gas. Heat added to a chemically closed system increases entropy by an amount greater than the ratio of the heat to the absolute temperature for an irreversible process entropy equals that ratio for a reversible process. Entropy is additive for a composite system. Reciprocal absolute temperature is defined as entropy change with energy. Equilibrium is reached at maximum entropy. The second law of thermodynamics is stated as the existence of an extensive function of state called the entropy that can only increase for an isolated system. Sekerka, in Thermal Physics, 2015 Abstract


 0 kommentar(er)
0 kommentar(er)
